Unlocking Precision Medicine: The Power of Gene Editing
Posted on 29-April-2024
1. Introduction
Since a few years ago, gene therapy has displayed potential in treating human diseases, and significant advancements have been made in its use. In order to treat disease or strengthen resistance to it, gene therapy involves either adding new genes or replacing damaged ones. One part of gene therapy is genome editing. The terms "gene editing" and "genome editing" refer to a similar procedure of precisely and specifically altering an organism's DNA (genetic material). Through this procedure, scientists can alter, add, or remove particular DNA segments in order to investigate gene function, rectify defects in genes, or produce desired features.
The discovery that a damaged DNA segment in a gene causes a cell's repair system to stitch the break back together is the foundation for genome editing. Researchers can replicate this spontaneous DNA repair process through genome editing. Mega nucleases, transcription activator-like effector nucleases (TALENs), and zinc-finger nucleases (ZFNs) are examples of advanced genome editing techniques derived from proteins. Clustered regularly interspaced short palindromic repeats, or CRISPR/Cas9, is an additional technique.
1.1. Gene Editing Technologies
The advent of highly adaptable genome-editing technologies in recent years has enabled researchers to quickly and affordably incorporate sequence-specific alterations into the genomes of a wide range of cell types and species. Zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9), and homing endonucleases or meganucleases are the core technologies that are currently most frequently used to facilitate genome editing.
- CRISPR/ Cas9
The term CRISPR (Clustered Regularly Interspaced Short Palindromic Repeat) illustrates how small, partially repeated DNA sequences in prokaryotes' genomes are arranged uniquely. Prokaryotes employ CRISPR and the protein it is linked with (Cas-9) as an adaptive immunity mechanism to protect themselves against viruses or bacteriophages. Due to its exceptional benefits, the CRISPR-Cas9 technology is a novel and useful approach to genome editing that has lately overtaken previous techniques. Thus, it has greatly advanced the comprehension of the principles behind gene diversity and gene editing and greatly benefited many branches of life science, including medicine, plant and animal breeding.
- ZFNs (Zinc- Finger Nucleases)
Targetable DNA cleavage reagents known as zinc-finger nucleases (ZFNs) have found widespread use as tools for targeting certain genes. Cellular DNA repair processes that result in both targeted mutagenesis and targeted gene substitution occur at impressively high frequency in response to ZFN-induced double-strand breaks. Numerous plants and animals can have their genomes altered with the use of zinc finger nucleases. ZFNs are also employed in the development of isogenic human illness models, a new class of genetic disease models. ZFNs' earliest therapeutic uses are probably going to be in ex vivo treatments that make use of the patient's own stem cells. The stem cells might be grown in vitro and then reintroduced into the patient to create differentiated cells with corrected functionality after the stem cell genome was edited.
- TALENs (Transcription Activator-Like Effector Nucleases)
Artificial restriction enzymes known as transcription activator-like effector nucleases (TALENs) has the capacity to cleave DNA upon entering into contact with a sequence of nucleotides. TALENs are useful for studying cell mutations in a variety of animals, including fish, xenopus, yeast, bacteria, rat embryonic stem cells, rodents, plants, and human cell lines. They are also useful for creating knockout strains. It is possible to assess the cutting efficiency of TALENs using a variety of assays.
Table 1. Differences between gene editing techniques
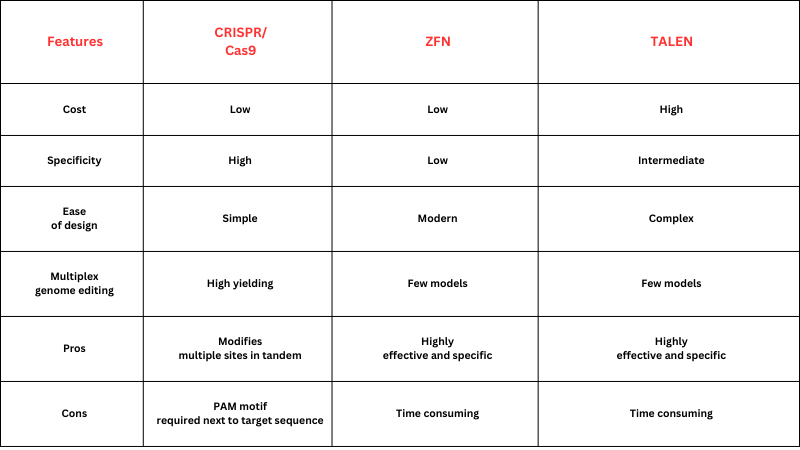
Source: National Centre of Biotechnology Information and Pragma Market Research Analysis
1.2. Gene Editing Applications
Applications for gene editing technologies are numerous and cover a number of industries, including biotechnology, medicine, agriculture, and fundamental research. Correcting or changing mutations that cause disease has great potential for treating genetic diseases through gene editing. In the medical industry, specific medicines for a variety of hereditary diseases, such as muscular dystrophy, sickle cell disease, cystic fibrosis, and certain cancers, can be created using gene editing methods.
Crops with improved qualities, such as higher yields, better nutritional value, and resistance to pests, illnesses, and environmental stresses, could be created by gene editing. Gene editing technologies have the potential to accelerate the breeding process and provide crops with desired traits more quickly than conventional breeding approaches by accurately altering the plant genome.
In both basic and applied studies, gene editing technologies are useful tools for understanding biological processes, disease mechanisms, and gene function. By using gene editing, scientists can develop novel therapeutic targets and therapies, as well as animal models of human diseases and insights into gene function.
Figure 1. Expansive Application
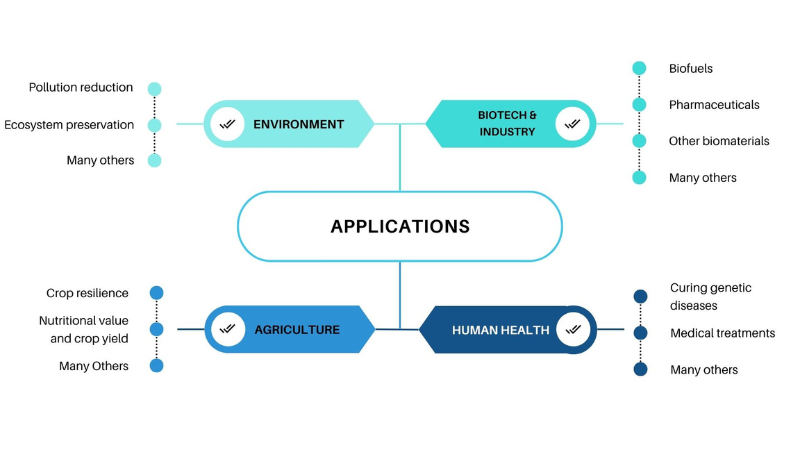
Source: Pragma Market Research Analysis
2. Overview
The definition of precision medicine is the evidence-based choice of an intervention targeted at enhancing disease prevention, diagnosis, or therapy according to the unique traits and preferences of the patient. The goal of a number of government and private sector programs and scientific developments is to elevate precision medicine to the forefront of patient care in the twenty-first century.
Although focused, customized treatments—like a new genome editing-based therapy—typically result in smaller sample numbers, it is crucial to take novel approaches into account when calculating benefit-risk. Clinical studies now use a single endpoint in their benefit-risk calculations to validate research results; additional data, such as patient preference, which is routinely gathered during the course of a clinical trial, is not taken into account. Project BENEFIT (Biostatistical Estimation of Net Effect For Individualization of Therapy) is presently conducting more research and validation on the statistical method to ascertain its viability for usage by precision medicine stakeholders.
It appears that precision medicine is a rigorous approach to healthcare, and that in order to be completely fulfilled, it would require the use of genomic data, new, innovative treatments, as well as additional tools and technologies.
The extensive use of gene editing in personalized medicine with rising pipeline therapies is anticipated to drive the growth. Also, the increasing usage of gene editing techniques in various fields with rising research and development activities is also one of the factors. While the stringent regulatory guidelines and high equipment costs for performing the procedures may restraining the growth.
2.1. History
When the history of genome engineering is examined in chronological sequence, it reveals an industry committed to using genetic research to benefit humankind. The organization has shown a dedication to making discoveries that have the potential to change the world, from designing the double helix to creating CRISPR modifications, all while abiding by strict rules and ethics.
Figure 2. Brief History of Gene Editing
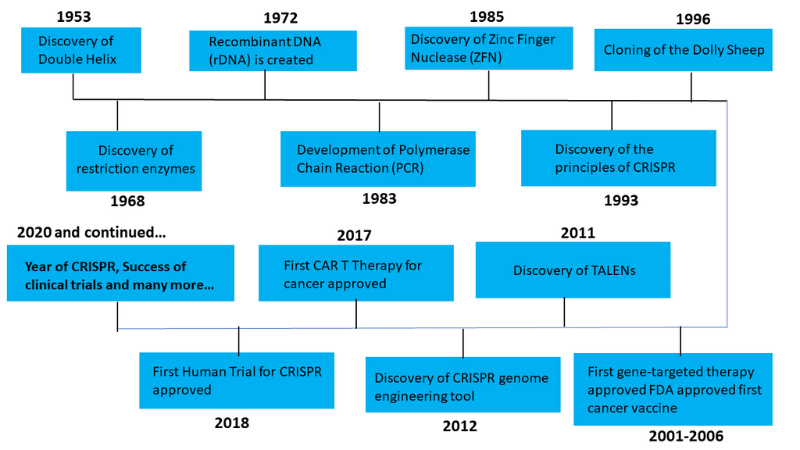
Source: Synthego and Pragma Market Research Analysis
2.2. Future Outlook
The possibility for gene editing to completely change the world is getting closer to reality as genetic technology advances at an incredible rate. Owing to the development of advanced tools such as CRISPR, scientists can now target and edit DNA with unprecedented precision. Numerous opportunities have arisen as a result, revolutionizing industries as diverse as healthcare, agriculture, and conservation.
Based on a naturally occurring bacterial trait, the researchers developed a delivery system utilizing protein engineering and artificial intelligence that can transport materials to human cells. Thus far, the research has demonstrated potential in eradicating cancer cells, and the approach is thought to alleviate a hindrance in developing effective treatments.
Additionally, it is anticipated that the combination of gene editing with personalized medicine will encourage innovation in drug development, making it easier to create safer, more effective drugs that are customized to each patient’s unique genetic profile. To ensure the appropriate and equitable implementation of new technologies, it will be necessary to carefully manage ethical and regulatory considerations as they develop.
3. Market Analysis
3.1. Segmentation
The segmentation for gene editing is given as below:
By Product & Service
• Reagents and Consumables
• Software & Systems
• Services
By Technology
• CRISPR
• ZFN
• TALEN
• Others
By End User
• Biotechnology & Pharmaceutical Companies
• Academic & Research Institutes
• Others
3.2. Key Players
Table 2. Companies involved in Gene editing
Sangamo Therapeutics, Precision BioSciences, GenScript, CRISPR Therapeutics, Horizon Discovery Ltd., Integrated DNA, Technologies, Inc., Cellectis, Editas Medicine, Takara Bio Inc., eGenesis, OriGene Technologies, Inc.
4. Recent Developments
Some of the developments and advancements of gene editing are mentioned as below:
• The VIB-UGent Center for Plant Systems Biology has applied for three separate field studies utilizing genome editing of maize, marking a significant advancement in the changing EU regulatory landscape. The goals of these CRISPR-Cas9 gene editing field trials are to improve plant tolerance to environmental stress, produce maize that is more digestible, and produce drought-resistant maize. The innovative work VIB has done in the field of genome editing should be a model for applying this technology to enhance sustainable agriculture. This project also emphasizes the need for more reasonable, scientifically grounded laws that take into account genome editing's special potential.
• In March 2024, Allogene Therapeutics, Inc. and Arbor Biotechnologies, Inc. announced licensing agreement to utilize Arbor's exclusive CRISPR gene-editing technology in Allogene's next-generation AlloCAR T platform, which is intended to treat autoimmune disease (AID). With the aim of reducing or eliminating the need for chemotherapy, Allogene has used its extensive knowledge of CAR T research and development to produce next-generation allogeneic CAR T experimental solutions. Early in2025, Phase 1 clinical trials for Allogene's first AID AlloCAR T experimental product are anticipated to begin.
• In November 2023, Vertex Pharmaceuticals Incorporated and CRISPR Therapeutics announced that CASGEVY (exagamglogene autotemcel [exa-cel]), a CRISPR/Cas9 gene-edited therapy, has been granted conditional marketing authorization by the Medicines and Healthcare Products Regulatory Agency (MHRA) of the United Kingdom (U.K.) for the treatment of sickle cell disease (SCD) and transfusion-dependent beta thalassemia (TDT).
• In October 2023, Intellia Therapeutics, Inc. and Regeneron Pharmaceuticals, Inc. recently announced an expansion of their research collaboration to develop additional in vivo CRISPR-based gene editing treatments targeted at neurological and muscle disorders. The partnership will utilize Intellia’s own Nme2 CRISPR/Cas9 (Nme2Cas9) systems, which are tailored for the delivery of viral vectors and intended to accurately alter a target gene, and Regeneron’s proprietary antibody-targeted adeno-associated virus (AAV) vectors and delivery systems.
• In September 2023, according to Genetic Engineering & Biotechnology News it was reported that Nickase is a revolutionary genome editing technology that creates many nicks in single DNA strands. This process is the basis of NICER. Two processes are used by the approach to correct heterozygous mutations: multiple nicks caused by Cas9 nickase and the use of a homologous chromosome as an endogenous repair template. The researchers propose that NICER greatly reduces unwanted mutations while being just as effective as CRISPR/Cas9.
• In August 2023, A new multi-million-dollar, five-year collaboration between Pairwise and Bayer will focus on breakthroughs in short-stature corn. This new initiative expands on the success of the firms' first five-year partnership for corn, soy, wheat, cotton, and canola by utilizing Pairwise's Fulcrum platform. The goal of Pairwise and Bayer's impending partnership is to improve and optimize gene-edited short-stature corn in preparation for its application in Bayer's Preceon Smart Corn System.
5. Regulatory Framework
Global differences in the regulatory framework surrounding gene editing are a reflection of differing ethical, cultural, and scientific perspectives. In many countries, government organizations and regulatory authorities concerned with ensuring the security and ethical application of these powerful tools closely monitor gene editing technology.
Gene-edited organisms are now considered genetically modified organisms (GMOs) in some regions, including the European Union, where they are also subject to stringent labelling rules and thorough risk evaluations. Some countries, like the US, have adopted a cautious approach, with regulatory agencies like the Food and Drug Administration (FDA) assessing gene editing products on an individual basis.
As gene editing technologies develop further while discovering novel applications, it will be increasingly vital to have uniform international laws that strike a balance between safety and ethics while promoting innovation. To create legal frameworks that support the ethical and responsible use of gene editing technologies while promoting global scientific advancement and innovation, cooperation between states and international organizations will be crucial.
6. Market Dynamics
6.1. Opportunities
O1: Expansion of gene therapy pipeline
O2: Growing focus on personalized medicine
6.2. Challenges
C1: Off targets and safety concerns
7. Conclusion
• The intersection between personalized medicine and gene therapy is a revolutionary frontier in healthcare. With the use of advanced technologies and genetic insights, personalized medicine offers the possibility to customize treatments.
• When taken together, these domains provide the door to a future in which healthcare is more accurate, effective, and individualized. But achieving this goal will need resolving significant ethical, legal, and scientific obstacles to make sure that these revolutionary technologies are applied accurately and ethically.
• The discovery of CRISPR brings a new direction for the gene editing technology and can be applied in majority of domain. It is proved to be very beneficial for cancer technology and treatment approaches. With more advanced features NICER is being developed which is more effective than CRISPR and it is continuously being explored.
• Focusing more towards the technology is is clear that safety and regulatory guidelines should be very strict when dealing with the genetic traits of the organisms. The regulatory agencies of different countries are amending the laws so that it cannot be mis leaded or misused by any organization as well as individual.
• Gene editing has the potential to transform healthcare in the future by providing new treatments and insights that could enhance human health and wellbeing.
With the unmatched accuracy that gene editing technologies like CRISPR-Cas9 offer in manipulating genetic material, they hold great promise for treating genetic diseases at their source. Combining gene editing with personalized medicine principles—which take into account each patient's unique genetic, environmental, and lifestyle factors—allows medical professionals to administer tailored treatments that optimize effectiveness while minimizing unwanted effects. In addition to offering people with diseases that were previously incurable hope, this combination of gene editing and personalized medicine opens the door for a future in which healthcare will be more accurate and individualized. Personalized medicine requires careful consideration of the ethical, regulatory, and societal consequences of gene editing to ensure responsible and equitable deployment.
DOWNLOAD PDF
